help with serial dilutions
You will be making some serial dilutions on Tuesday - I have noticed that these tend to puzzle some of you!
A serial dilution is a dilution performed several times in a row, so that you end up with a set of solutions, where each solution is less concentrated than the previous solution.... perhaps this sounds cryptic, so here is what I mean in a diagram:
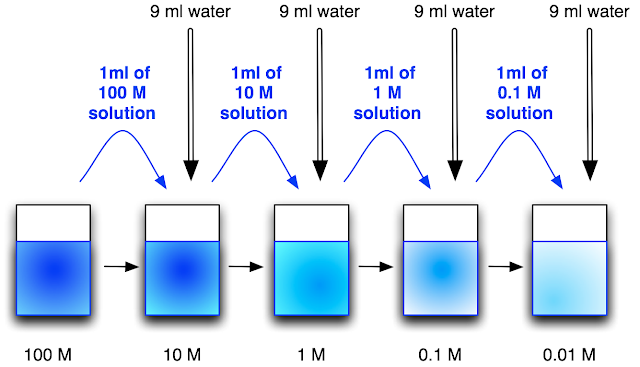
So, in the diagram above there is a set of solutions, and each solution is 10 x more dilute than the previous solution. This would be the result of a serial dilution.
So how was this serial dilution performed in order to get this set of solutions?
Well, some of the 100 M solution would have been pipetted into a new container and then diluted 10 x to make the 10 M solution. Then, some of the 10 M solution would have been pipetted into a new container and then diluted 10 x to make the 1 M solution and so on...
In this example, seeing as it is a 10 x dilution, this means each time you need to take 1 part of what you are diluting, and mix this with 9 parts of water. i.e. 1 ml into 9 mls, or 10 mls into 90 mls or 0.1ml into 0.9 mls ..... The volumes used for these dilutions would depend on your experiment and what volumes you need to have at the end of the day of each of the dilutions.
So in my example I have chosen to do my 10 x serial dilutions by taking 1 ml solution to be diluted and adding to this 9 mls of water, so each time I end up with 10 ml total:
Of course, serial dilutions don't have to be 10 x dilutions (which could be written as 1:9), they could be 2 x (which could be written as a 1:1 dilution), 3 x (which could be written as a 1:2 dilution), 4 x (which could be written as a 1:3 dilution) etc
I hope that helps you out
A serial dilution is a dilution performed several times in a row, so that you end up with a set of solutions, where each solution is less concentrated than the previous solution.... perhaps this sounds cryptic, so here is what I mean in a diagram:
So, in the diagram above there is a set of solutions, and each solution is 10 x more dilute than the previous solution. This would be the result of a serial dilution.
So how was this serial dilution performed in order to get this set of solutions?
Well, some of the 100 M solution would have been pipetted into a new container and then diluted 10 x to make the 10 M solution. Then, some of the 10 M solution would have been pipetted into a new container and then diluted 10 x to make the 1 M solution and so on...
In this example, seeing as it is a 10 x dilution, this means each time you need to take 1 part of what you are diluting, and mix this with 9 parts of water. i.e. 1 ml into 9 mls, or 10 mls into 90 mls or 0.1ml into 0.9 mls ..... The volumes used for these dilutions would depend on your experiment and what volumes you need to have at the end of the day of each of the dilutions.
So in my example I have chosen to do my 10 x serial dilutions by taking 1 ml solution to be diluted and adding to this 9 mls of water, so each time I end up with 10 ml total:
Of course, serial dilutions don't have to be 10 x dilutions (which could be written as 1:9), they could be 2 x (which could be written as a 1:1 dilution), 3 x (which could be written as a 1:2 dilution), 4 x (which could be written as a 1:3 dilution) etc
I hope that helps you out

Comments
Post a Comment