all those different units for concentrations...
In your next 107 practical and your next 109 maths workshop you are going to be dealing with concentrations, and after that concentrations are going to keep popping up, so it would be good for you to know about the various ways we can talk about concentration...
Let's start with a definition:
Let's start with a definition:
Concentration
= The amount of a specified substance in a unit amount of another substance
There are various ways concentrations can be expressed....
1. As the weight of something per unit volume
For example, grams per litre.
i.e.
20 grams per litre (written as 20 g l-1) means there are 20 grams of substance in one litre of solution.
or
34 milligrams per litre (written as 34 mg l-1) means there are 34 milli grams of substance in one litre of solution (this is the same as saying 0.034 grams per litre)
2. As a percentage concentration
There are three ways percentage concentration can be used:
There are three ways percentage concentration can be used:
| Percentage Concentration | Meaning |
| % v/v volume per volume | used where both chemicals are liquids (eg if I dilute 50 mL of acetic acid by adding it to 50 mL of water there is now 50 mL of acetic acid in a total volume of 100 mL, hence the acetic acid concentration is now 50% v/v) |
| % w/v weight per volume | used where a solid chemical is dissolved in liquid (eg if I dissolve 10 g of table salt, sodium chloride, to make up a total volume of 100 mL of solution then I have made a 10% w/v solution of sodium chloride) |
| % w/w weight per weight | used where the weight of each chemical is used and not the volume (eg If I dissolve 10 g of fat in 90 g ethanol so the total mass of the whole solution is 100 g, then I have made a 10% w/w solution of fat) |
table taken from: http://toolboxes.flexiblelearning.net.au/demosites/series4/412/laboratory/personalstudy/PSPerVolWt.htm
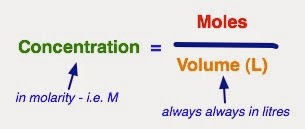
3. As a Molarity
Molarity (M) is the number of moles in a litre. i.e. a 1 M NaCl solution has 1 mole of NaCl in one litre.
(see my post on moles if you are unsure about moles!)
NOTE: Molarity is ALWAYS moles per LITRE!!!
To find your molarity you do moles divided by volume (in litres!!):
You can use prefixes with molarity, i.e. 1 M is the same as 1000 mM ....
It is a good idea to try to understand the different ways concentration can be written, you will need to be able to work with concentrations all through your degree and beyond!
Comments
Post a Comment